Medical network - on February 10, as the beginning of the "two votes" national version of the plan and promoting press disease related policies, 2017 is destined to be extraordinary one year. The personage inside course of study analysis, under the current compliance requirements, foreign capital is not willing to get in, as a multinational drug companies, once being punished in China, in a foreign country can be joint punishment, the consequences will be severe. And after sales outsourcing, enterprise risk is much smaller.
In fact, since 2015, the foreign drug companies have been by spinning off non-core business to reduce costs. More and more foreign companies with respect to its some product lines in China, with Chinese pharmaceutical companies conducting business cooperation. This is lilly, novartis, GSK, etc., in the last year has some varieties distribution rights to one of the important reasons for the local operators. Could see from this, the foreign and domestic drug firms business cooperation in the form of is the trend of The Times in 2017.
The acquisition of domestic companies from foreign product line of mature product
Astrazeneca and Kang Zhe pharmaceutical industry, which was signed on February 26, 2016, exclusive license agreement, Kang Zhe pharmacy will pay $310 million to astrazeneca, to gain plendil commercially licensed product exclusive rights in China. Kang Zhe, meanwhile, is the first big shareholder of a-share listed companies Tibetan pharmaceutical subsidiary Everest Future $190 million purchase of astrazeneca in's product, the brand on A global scale (except the United States) related assets.
On May 19, 2016, tai ling pharmaceutical group announced agreement with novartis, to novartis acquired international well-known brand of orthopedic dense cover rate, relevant intellectual property rights, licenses and other assets, trading at $145 million.
On July 4, 2016, glaxosmithkline (GSK) announced the transfer of its holdings of China nanjing meirui pharma, meirui factory, and with three kinds of urinary products (shed pavilion quick release tablets, pavilion zyban and the completed) related to the local production and supply of the business will be under the existing legal entities by KaiDeSi of operations.
On October 11, 2016, junior signed with astrazeneca pharmaceutical wholly owned subsidiary of Hong Kong junior exclusive license agreement, astrazeneca agreed to grant the Hong Kong stroke in China on diabetes drug Byetta (Essex that peptide injection) and Bydureon (asay the slow-release preparation of peptide) commercially exclusive rights.
On November 9, 2016, Shanghai - lilly announced that China has hundred million teng medicine exclusive agreement with Chinese medicine company, its products of antibiotics and carved lowe and steady reliable in mainland China within the scope of the promotion and distribution rights in January 1, 2017 formally awarded billions of medicine.
Why the domestic pharmaceutical companies will spent heavily on "buying"
Felodipine and in accordance with Sam
Felodipine (felodipine zyban) by astrazeneca former research production, for the treatment of high blood pressure and stable angina, belongs to the national health insurance b class of drugs. In accordance with the's (isosorbide mononitrate zyban), developed by astrazeneca to ischemia therapy of coronary heart disease, in Europe in 1985, sold more than 40 countries around the world, outside the United States market sales of nearly $57 million in 2015.

The figure 1 shows that the los to peaceful isosorbide mononitrate nearly two years in China's urban public hospitals, public hospitals at the county level, urban community patterns in towns and townships of four chemical medicine and have a good sales, including felodipine two years in the four major chemical medicine market combined sales of more than 1 billion yuan. Billions of varieties, sales volume is large enough, for deep inroads into the existing hospital channel, itself is the value of enough.
After Kang Zhe pharmaceutical flange, from the point of view of product line, felodipine and the company now sales coverage, hospital sources have 50% of the city is a coincidence, complementary to the existing line of heart head blood-vessel market, can quickly integrate into the company's existing product line, bring benefit to the company.
Dense cover rate
Novartis branded dense cover rate (salmon calcitonin) belong to the health care class b drugs, mainly used in the treatment of osteoporosis, hypercalcemia. As the orthopaedic field worldwide well-known brand, cover rate series products for a long time to maintain the stable sales income, and has a global sales network.

The meters data shows that in the past two years salmon calcitonin in China's urban public hospitals, public hospitals at the county level, urban communities and rural hospitals four chemical medicine pattern combined sales of more than 300 million yuan. Our country has 21 million osteoporosis patients, only more than 140 received treatment, broad market space.
Acquisition of dense cover rate brand, will rich tai ling pharmaceutical existing tumors, central nervous, four product lines in the field of treatment for liver disease and respiratory system, strategic into the orthopaedic field. After the transition will bring to the company significant revenue and profits.
S pavilion quick release tablets, pavilion zyban and the completed
Shed, kiosks, nanjing meirui pharmaceutical co., LTD. Quick release tablets (tartaric acid tottenham constant speed release), shed his pavilion zyban (tartaric acid tottenham luo certain zyban) and the completed (naphthalene in sheet) for the three urinary products, are health b drug, which shed, pavilion zyban for sole varieties.
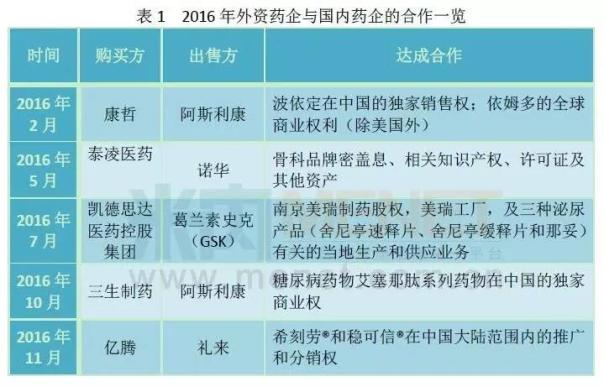
Luo certain can be seen from figure 3, tottenham and naphthalene in public hospitals in the city, the public hospitals at the county level, the urban community and the structure of towns and townships of four chemical medicine combined sales.
KaiDeSi medicine holding group in the field of antibiotic market, market areas, liver disease of heart head blood-vessel, products have the layout of the tumor and the nanjing meirui pharmaceutical co., LTD. Of urological product portfolio will no doubt fill KaiDeSi of existing business of blank in this field.
Essex that peptide
Byetta (Essex that peptide injection) as astrazeneca exclusive varieties, to improve glycemic control in patients with type 2 diabetes, FDA approval in 2005, got the CFDA approved in 2009. The slow-release preparation Bydureon for Essex that peptide, used for blood glucose control in patients with type 2 diabetes.
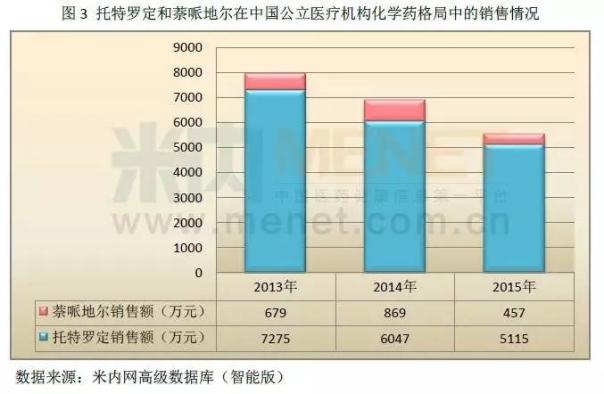
The figure 4 shows that from 2014 to 2015 Essex that peptide in public hospitals in the city, the public hospitals at the county level, the urban community and in towns and townships of four chemical medicine pattern combined sales increased year by year, sales reached one hundred million yuan in 2015, two years remained at more than 75% growth rate.
The strategic cooperation with astrazeneca, make a good pharmaceutical product line in the field of diabetes this major chronic diseases has been effectively develop and market penetration. Companies will move into diabetes industry, the plate can be a company another driver of growth in the future.
He carved credible ® ® and stability
He carved lowe ® (cefaclor zyban (Ⅱ)), entered China in 1993, and become the domestic doctors high recognition brand of oral antibiotics. Steady reliable ® (vancomycin hydrochloride for injection) to enter the Chinese market in 1996, due to its excellent efficacy and good safety, become the treatment of MRSA (methicillin resistant staphylococcus aureus) the gold standard of infection.
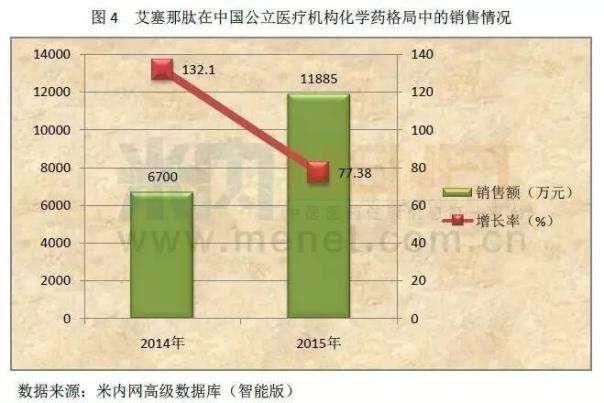
According to m, according to data from the network of vancomycin and cefaclor in almost three years in China's urban public hospitals, public hospitals at the county level, urban community and four chemical medicine pattern in towns and townships, total sales of one hundred million yuan breakthrough, vancomycin in 2015, the total sales of nearly 1 billion yuan. The strategic cooperation will undoubtedly increase its content in the field of anti-infective ability, and further enhance the core competitiveness of hundred million teng medicine.
conclusion
Boom in 2017, this form of cooperation will continue, and we look forward to the emergence of a "buying"! |
