Medical network February 10th hearing of breast cancer is one of the major diseases that endanger women's health, over the years has been the first female cancer incidence of a class of diseases. Statistics show that over the past 10 years, with the rapid development of global industrialization, as well as the aging of the population and people attach great importance to physical examination screening, the incidence of cancer statistics has more than doubled.
The treatment of breast cancer is surgery combined with chemotherapy and radiotherapy, with the advent of new targeted therapies, patients have more choices.
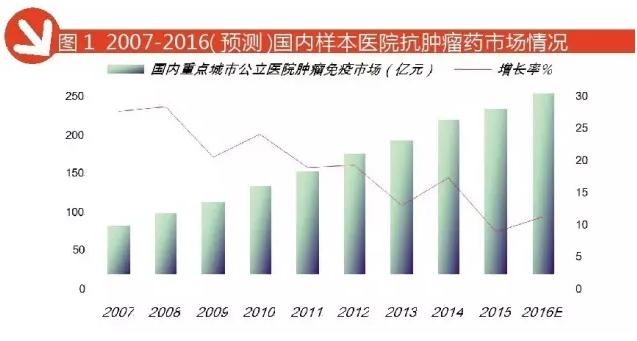
One hundred billion yuan anti-tumor Market
According to the "Chinese meters within the network of public hospitals in the city of chemical drugs terminal monitoring and analysis system (HDM system) data, in 2015 the city anti tumor public hospital, county public hospitals, city community, township hospitals, pharmacies and three entities shop terminal 6 drug market reached 95 billion yuan, an annual growth rate of 13.53%. Conservative forecast in 2016 China's three major markets in the terminal market will reach $105 billion, an increase of 10.52% over the previous year.
According to HDM data, 2016 1-3 quarter of the amount of anti tumor domestic key city public hospital and immunomodulator drug sales reached 17 billion 684 million yuan, forecast for the year 2016 will reach 23 billion 600 million yuan, year-on-year growth of 9.07%, antineoplastic drugs accounted for nearly 60%, an important position. Among them, the breast cancer drug TOP 5 paclitaxel, docetaxel, capecitabine, trastuzumab and leuprolide, accounting for 56.37%.
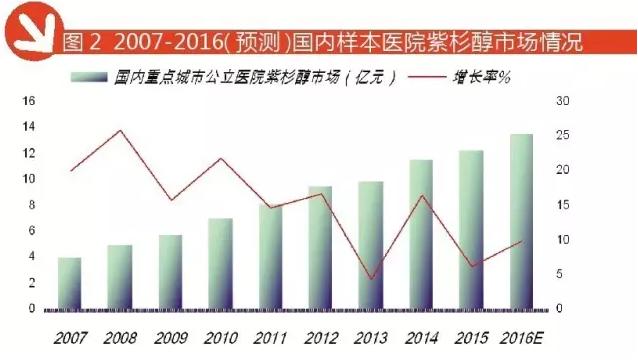
 Paclitaxel: TOP 3 manufacturers accounted for 85%
Paclitaxel is a broad-spectrum anticancer drug, and its unique microtubule stabilizing effect is widely used in the chemotherapy of a variety of common malignant tumors. The drug is a "double-edged sword", which can cause allergic reactions, nephrotoxicity, neurotoxicity and adverse effects on cardiovascular toxicity in the composite solvent effect, which brings new harm to patients. In order to give full play to the anticancer effect of paclitaxel, people have made a deep exploration of the new formulation, and the breakthrough in the treatment of paclitaxel liposome has been made.
Paclitaxel liposome has high safety, good tolerance and passive targeting of tumor tissues and lymph nodes. The distribution of paclitaxel liposome is higher than 2 to 23 times. Passive targeting is the basic characteristic of liposome intravenous administration. These characteristics make paclitaxel liposome has the advantage of anti lymph node metastasis, but also reduce its toxic effects on the heart and kidney, but more importantly, paclitaxel liposome as well as tumor targeting.
Compared with the traditional paclitaxel preparation, paclitaxel liposome has the advantages of fast distribution and slow elimination. In particular, the concentration of paclitaxel liposome in tumor tissues and lymph nodes is higher, and it has stronger anti-tumor effect and anti lymphatic metastasis.
According to HDM system data, the 2016 1-3 quarter of domestic public hospitals in major cities in the amount of paclitaxel sales amounted to 1 billion 15 million yuan, the forecast for the full year in 2016 the amount of paclitaxel was $1 billion 354 million, an increase of 9.96% over the previous year. TOP 3 is CISCO manufacturers: leaves occupy 60.73%, Fresenius Cabi accounted for 14.17%, /Corden accounted for 10.49% of boomi Bristol myers.
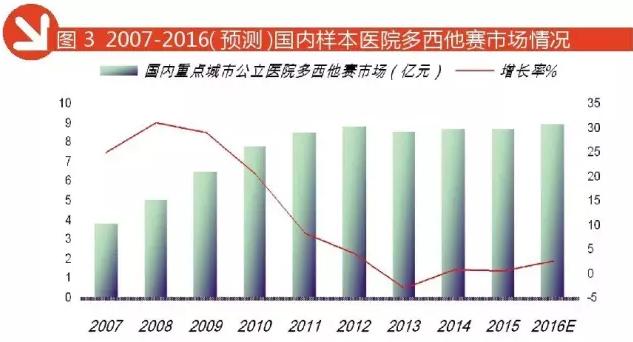
 Docetaxel: coveted dominance of time
Docetaxel belongs to the inhibitor of microtubule depolymerization (Docetaxel, docetaxel), which belongs to the taxanes, by Planck Le Rhone company first developed and used in the treatment of advanced breast cancer and non-small cell lung cancer. Found in phase II clinical trials conducted at the M.D.Anderson cancer center, has a good effect on the application of docetaxel cisplatin and carboplatin failure of patients with advanced ovarian cancer. And docetaxel on prostate cancer, pancreatic cancer, soft tissue tumor, head and neck cancer, gastric cancer, esophageal cancer and other solid tumors showed significant activity.
Docetaxel has listed has been in the United States, Japan and other more than and 80 countries in 1996, China began to enter clinical verification after listing. Jiangsu Hengrui development has been in the domestic market, at present the hospital in Henry docetaxel sales have more than imported drugs, become the biggest selling businesses.
According to HDM data, 2016 1-3 quarter of the amount of domestic key city public hospital docetaxel sales amounted to 675 million yuan, in 2016 the annual forecast of docetaxel medication amounted to 899 million yuan, year-on-year growth of 2.79%, much higher than similar varieties. Although the total sales behind docetaxel and paclitaxel, but the rapid growth, catch up with paclitaxel a similar drug in point the day and await for it. In 2016 the domestic key city public hospital 5 TOP docetaxel manufacturers as Jiangsu Hengrui occupy 40.72%, Sanofi occupy 29.74%, Shandong Qilu occupy 15.56%, Shenzhen Wanle occupy 8.39%, aosaikang occupy 2.04%.
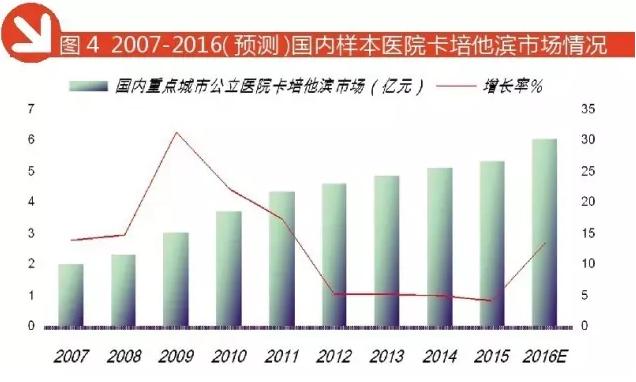
 Capecitabine: Roche exclusive 70% share
Shanghai Roche Capecitabine Tablets has entered the national health insurance directory of drugs, called Shiroda, Capecitabine Tablets is a first-line treatment of colorectal cancer, breast cancer combined chemotherapy and metastatic gastric cancer.
Normal and tumor cells can metabolize 5-FU 5- fluoride -2- deoxyuridine monophosphate (FdUMP) and 5- three (FUTP) of floxuridine phosphate. Metabolites are involved in two different mechanisms. Studies have shown that capecitabine has an effect on human peripheral blood lymphocytes in vitro.
Capecitabine is the preferred first-line chemotherapy for patients with metastatic colorectal cancer, and the survival time of capecitabine combined with other drugs is superior to 5-FU/LV monotherapy.
According to HDM data, 2016 1-3 quarter of domestic key city public hospital capecitabine sales amounted to 458 million yuan, forecast for the year 2016 public hospital capecitabine sales amounted to 611 million yuan, year-on-year growth of 13.66%, capecitabine and docetaxel combined with treatment of anthracycline containing chemotherapy the failure of metastatic breast cancer. In 2016 the domestic key city public hospital capecitabine 4 TOP manufacturers, Roche occupy 72.64%, Jiangsu Hengrui occupy 10.81%, Qilu pharmaceutical cttq accounted for 9.15%, accounted for 7.40%, showing a high degree of concentration of the situation.
 Trastuzumab: Herceptin monopolize the domestic market
Trastuzumab by American gene Tektronix Inc successfully developed in September 25, 1998 by the U.S. FDA approved for clinical use, marketed as Herceptin (Hessaitin). Roche's Genentech is after the merger, Roche's drug monoclonal antibody. Targeted gene therapy compared with conventional radiotherapy, chemotherapy and hormone therapy for breast cancer trastuzumab, can choose to kill tumor cells by gene, without affecting the normal human tissue cell survival.
Trastuzumab is a derivative of a recombinant humanized DNA monoclonal antibody, is representative of the anti tumor targeting antibody drugs. The drugs can selectively act on human epidermal growth factor receptor -2 extracellular region, combined with its specific receptor, signal transfer effect of growth; and to promote the internalization of the receptor protein degradation, through the aggregation of immune cells to attack and kill tumor cells, for clinical treatment of advanced breast cancer. At present, the EU has approved Herceptin and docetaxel combined with human epidermal growth factor receptor 2 (HER2) positive metastatic breast cancer as first-line therapy in combination.
In 2002, trastuzumab has been introduced to our hospital market, under the name of Herceptin listed. At present, the domestic market by trastuzumab Herceptin exclusive.
According to HDM data, 2016 1-3 quarter of domestic key city public hospital trastuzumab treatment amounted to 441 million yuan, forecast in 2016 China's key city public hospital trastuzumab treatment amounted to 588 million yuan, year-on-year growth of 11.07%. Herceptin drug with low toxicity, the treatment effect was obvious, prolonged survival, is of great significance to improve the quality of life of patients with advanced cancer, is the power of McAb market driven growth.
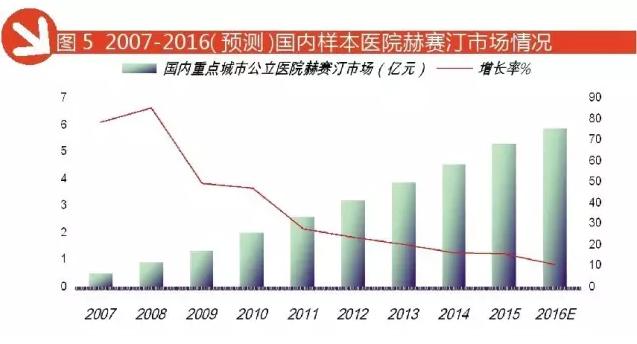 Leuprorelin: to stabilize a situation of tripartite confrontation pattern
Japan is a major manufacturer of Takeda global leuprolide, the name Lupron, which dominate the global market about half of leuprolide. It is reported that Takeda and Abbott co founded TAPP (TAP) held by the pharmaceutical company's patent expired in 2004. Two companies 33 years earlier in 2010, the company expires, TAPP leuprorelin operated by Abbott Company, in addition, Tailai, Senauer Faye Ann Wandt, Orion, Ann MediGene, OLT is also selling leuprolide manufacturers. In recent years, the rise in medical consumption situation, leuprolide has gradually been accepted domestic doctors and patients.
At present, Alarelin polypeptide drugs still occupy the foreign and joint ventures, higher prices for domestic patients unable to bear, thus restricting the development of the market. With the improvement of the national health care system, this situation is expected to be improved. LHRH analogues are used not only in the treatment of breast cancer, but also for prostate cancer and endometriosis. From the overall market, the sales of drugs increased year by year. With the increase of the incidence of breast cancer and the improvement of people's knowledge of breast cancer prevention, the endocrine therapy market will be expanded. Anti estrogens and aromatase inhibitors are dominant in endocrine therapy for breast cancer, especially the aromatase inhibitors increased rapidly in recent years, has become a hotspot in the field.
Leuprorelin is drugs ranked first in the domestic key city public hospital in Judeline class of prescription drugs, but also the highest growth rate of varieties. This class of peptide drugs in general dose is very small, but the need for long-term administration, which provides an opportunity for innovative formulations. Leuprorelin sustained-release injection Takeda pharmaceutical company is at the end of last century our country enters the clinical drug, marketed as enantone. Then the original research laboratories developed sustained-release microsphere technology, this technology is the drug encapsulated in the microspheres, administered by subcutaneous or intramuscular, slow the release of the drug, the change process of transport in vivo, prolong the action time. In 2009, Beijing boente pharmaceutical, Shanghai Lizhu pharmaceutical leuprorelin injection listed microspheres approved.
According to HDM data, 2016 1-3 quarter, the domestic key city public hospital leuprorelin treatment amounted to 296 million yuan, forecast for the year 2016 city public hospital leuprorelin treatment amounted to 394 million yuan, year-on-year growth of 29.74%. TOP 3 manufacturers: Tianjin Takeda occupy 53.37%, Beijing boente pharmaceutical industry accounted for 23.68%, accounted for 22.95% of the Shanghai franc. Basically formed a domestic leuprorelin drug market a situation of tripartite confrontation pattern.
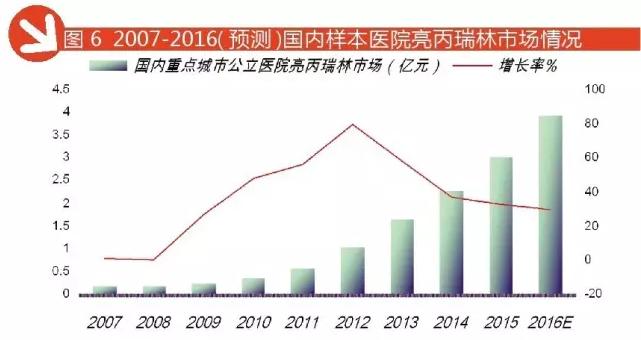 |
